1. A student set up an electrolysis experiment using a solution of sodium chloride (NaCl) and graphite electrodes. After running the experiment, the student noticed a yellow-green gas evolving at the anode. What could be the gas produced?
a) Hydrogen gas
b) Chlorine gas
c) Oxygen gas
d) Nitrogen gas
Answer: b) When electrolysis is conducted using a solution of sodium chloride (NaCl), at the anode, chlorine gas (Cl2) is produced. This is due to the discharge of chloride ions (Cl-) from the solution, which undergoes oxidation to form chlorine gas.
2. An investigation was carried out to assess the conductivity of liquids. Four solutions were sequentially added to a beaker, and the conductivity was tested using a circuit comprising a bulb, a battery, and connecting wires as illustrated in the setup below. The bulb glowed brightly in when solution X and dimly in solution Y. Solutions Z and W failed to conduct electricity. What conclusion can be drawn from this experiment?
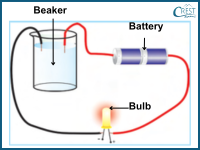
a) Solution X is the least conductive.
b) Solution Y is the most conductive.
c) Solutions Z and W are the same.
d) Solution Y contains the most impurities.
Answer: d) The observation that the bulb glows brightly in solution X and dimly in solution Y indicates that solution X is a better conductor of electricity than solution Y. Since solutions Z and W failed to conduct electricity, it can be inferred that they are both poor conductors.
3. Imagine you have a brass key that you want to electroplate with a layer of silver. Which metals should the cathode and anode be made of in this electroplating setup?
a) Cathode: Silver, Anode: Brass
b) Cathode: Brass, Anode: Silver
c) Cathode: Copper, Anode: Zinc
d) Cathode: Zinc, Anode: Copper
Answer: b) The anode is the object that is being electroplated, so it should be made of the material you want to deposit (silver in this case). The anode is the source of the plating material, so it should be made of the material that you're plating onto the cathode (brass in this case). This ensures that the silver layer is deposited onto the brass key, achieving the desired electroplating process.
4. An electric fan consumes a total charge of 960 C in 4 minutes. Calculate the average current through the fan.
a) 240 A
b) 4 A
c) 6 A
d) 40 A
Answer: b) To calculate the average current (I) through the fan, the formula used is:
I = Q/t
where
Q = Total electric charge = 960 C
t = Time = 4 minutes = 240 seconds
Substitute the values into the formula:
I = 960/240
I = 4 Amperes (A)
So, the average current through the fan is 4 A.
5. In the following question, you will find an assertion and a reason. Select the appropriate option that applies.
Assertion: LED bulbs can be used to test the electrical conductivity of liquids.
Reason: LED bulbs are sensitive to even small amounts of electric current and can indicate whether a liquid is a conductor.
a) Both the assertion and reason are correct, and the reason explains the assertion.
b) Both the assertion and reason are correct, but the reason does not explain the assertion.
c) The assertion is correct, but the reason is incorrect.
d) The assertion is incorrect, but the reason is correct.
Answer: a) The reason given supports the assertion by explaining that LED bulbs are sensitive to even small amounts of electric current. This sensitivity allows them to detect whether a liquid is a conductor of electricity. If the LED bulb lights up when placed in a liquid, it indicates that the liquid is conducting electricity and therefore is a conductor.


