1. The neutral atom 'Y' has the electronic configuration 2, 8, 5. What is the electronic configuration of the ion Y+?
a) 2, 8, 5
b) 2, 8, 6
c) 2, 8, 4
d) 2, 8, 7
Answer: c) The neutral atom 'Y' has the electronic configuration 2, 8, 5. When an atom loses one electron to become a Y+ ion, the electronic configuration of the ion changes as follows:
Neutral Atom 'Y': 2, 8, 5
Y+ Ion: 2, 8, 4
2. An atom has an atomic number of 17 and a mass number of 35. How many protons, neutrons, and electrons does this atom have?
a) Protons: 17, Neutrons: 35, Electrons: 17
b) Protons: 17, Neutrons: 18, Electrons: 17
c) Protons: 35, Neutrons: 17, Electrons: 18
d) Protons: 18, Neutrons: 17, Electrons: 17
Answer: b) Given,
Atomic number (Z) = 17
Mass number (A) = 35
The atomic number (Z) represents the number of protons in an atom. Therefore, the number of protons is 17.
The mass number (A) represents the sum of protons and neutrons in the nucleus. Therefore, the number of neutrons can be calculated as:
Neutrons (n) = Mass number (A) - Atomic number (Z)
Neutrons (n) = 35 - 17 = 18
For a neutral atom, the number of electrons is equal to the number of protons. Therefore, the number of electrons is also 17.
3. Consider the number of protons and neutrons in the following elements.
Element 1: 7 protons, 8 neutrons
Element 2: 8 protons, 7 neutrons
Element 3: 7 protons, 9 neutrons
What can you conclude about this information?
a) Elements 1 and 2 are isobars.
b) Elements 1 and 2 are isotones.
c) Elements 1 and 3 are isotopes.
d) Elements 1, 2, and 3 are isobars.
Answer: c) Isotopes are atoms of the same element that have the same number of protons (which defines the element) but different numbers of neutrons in their atomic nuclei.
Elements 1 and 3 have the same number of protons (7), which means they are the same element. However, they have different numbers of neutrons (8 and 9), making them isotopes of the same element.
Element 2 has a different number of protons (8) compared to Elements 1 and 3, indicating that it is a different element altogether.
4. Select the name of the experiment depicted in the image and accurate labels for the components labelled as A, B, C, and D.
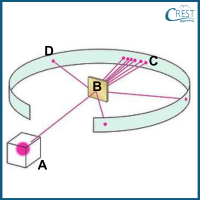
a) Experiment: Rutherford scattering experiment,
A: Source of β-particles, B: gold foil, C: deflected particles, D: non-deflected particles
b) Experiment: Thomson Scattering Experiment,
A: Source of α-particles, B: gold foil, C: non-deflected particles, D: deflected particles
c) Experiment: Thomson Scattering Experiment,
A: Source of β-particles, B: silver foil, C: non-deflected particles, D: deflected particles
d) Experiment: Rutherford Scattering Experiment,
A: source of α-particles, B: gold foil, C: non-deflected particles, D: deflected particles
Answer: d) Experiment: Rutherford Scattering Experiment,
A: Source of α-particles, B: gold foil, C: non-deflected particles, D: deflected particles
5. Consider the elements X, Y, and Z with the following atomic number:
X: 14
Y: 17
Z: 10
Which element has the highest valence electrons?
a) X
b) Y
c) Z
d) They all have the same number of valence electrons.
Answer: c) Valence electrons are the electrons in the outermost energy level of an atom. Among the provided elements:
Element X (atomic number 14) has 4 valence electrons.
Element Y (atomic number 17) has 7 valence electrons.
Element Z (atomic number 10) has 8 valence electrons.
Therefore, element Z has the highest number of valence electrons.


